1. Μitochondrial thioredoxin 2 may use shared or distinct areas for covalent and non-covalent interactions with its protein ligands.
1Department of Chemistry, University of Patras, 26504 Rion, Greece
2Institute of Chemical Biology, National Hellenic Research Foundation, Vas. Constantinou 48, 11635 Athens, Greece
* Author to whom correspondence should be addressed.
 Antioxidants 2024, 13(1), 15; https://doi.org/10.3390/antiox13010015
Antioxidants 2024, 13(1), 15; https://doi.org/10.3390/antiox13010015Submission received: 1 November 2023 / Revised: 9 December 2023 / Accepted: 16 December 2023 / Published: 20 December 2023
(This article belongs to the Special Issue Interactions of Redox-Active Proteins and Their Substrates)
Abstract
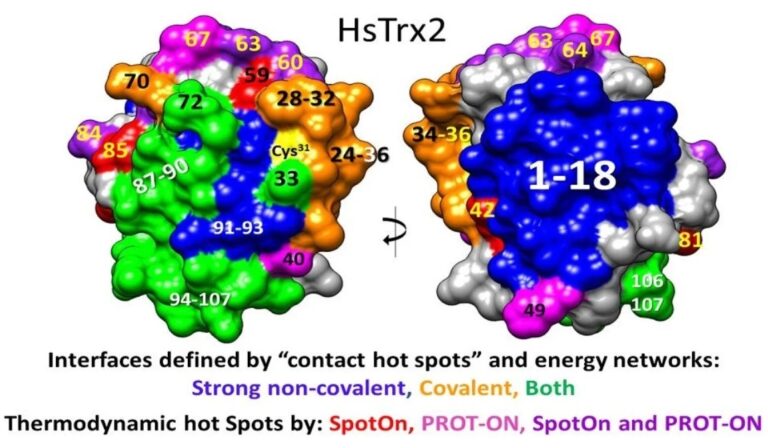
In silico approaches were employed to examine the characteristics of interactions between human mitochondrial thioredoxin 2 (HsTrx2) and its 38 previously identified mitochondrial protein ligands. All interactions appeared driven mainly by electrostatic forces. The statistically significant residues of HsTrx2 for interactions were characterized as “contact hot spots”. Since these were identical/adjacent to putative thermodynamic hot spots, an energy network approach identified their neighbors to highlight possible contact interfaces. Three distinct areas for binding emerged: (i) one around the active site for covalent interactions, (ii) another antipodal to the active site for strong non-covalent interactions, and (iii) a third area involved in both kinds of interactions. The contact interfaces of HsTrx2 were projected as respective interfaces for Escherichia coli Trx1 (EcoTrx1), 2, and HsTrx1. Comparison of the interfaces and contact hot spots of HsTrx2 to the contact residues of EcoTx1 and HsTrx1 from existing crystal complexes with protein ligands supported the hypothesis, except for a part of the cleft/groove adjacent to Trp30 preceding the active site. The outcomes of this study raise the possibility for the rational design of selective inhibitors for the interactions of HsTrx2 with specific protein ligands without affecting the entirety of the functions of the Trx system.
2. Towards a Holistic Understanding of the Interactions of Redox-Active Proteins.
Division of Organic Chemistry, Biochemistry and Natural Products, Department of Chemistry, University of Patras, 26504 Rion, Greece
 Antioxidants 2024, 13(11),1306; https://doi.org/10.3390/antiox13111306
Antioxidants 2024, 13(11),1306; https://doi.org/10.3390/antiox13111306Submission received: 21 October 2024 / Accepted: 25 October 2024 / Published: 28 October 2024
(This article belongs to the Special Issue Interactions of Redox-Active Proteins and Their Substrates)